For millions of people, anxiety feels like a storm that starts and ends in the mind, a relentless cycle of worry and racing thoughts. However, a revolutionary shift in modern science suggests we may be looking in the wrong place for the origins of our deepest emotions.
Emerging research is revealing that our mental state is actually an intricate biological conversation happening between the brain’s immune system, the integrity of the digestive tract, and the trillions of microbes living within us. Biological signals between brain immune cells, gut barriers, and the microbiome interact to regulate anxiety and social fear through the switchboard.
Mapping the ‘Anxiety Switchboard’ allows researchers to identify how immune cells known as microglia act as biological regulators, capable of turning anxiety-like behaviors on or off. Research confirms that the health of our gut lining serves as a critical barrier, where even small disruptions send inflammatory signals to the brain that directly reshape fear processing. Gaining insight into these hidden biological signals may provide the key to achieving a calmer nervous system and more resilient well-being.
Current progress builds on existing work identifying the holistic brain-gut connection and the enteric nervous system, which functions as a ‘second brain’ to shape mood. While these discoveries are still early, they may pave the way for a new generation of mental health therapies that strengthen the body’s internal balance instead of simply altering brain chemistry. Here is what scientists have recently found, what it really means, and how this evolving research could eventually influence the way we understand mental wellness.

Core Breakthroughs in Modern Brain–Gut Signaling Research
- Brain Immune “Switchboard”: Research identifies how two distinct types of microglia act like accelerator and brake pedals for anxiety-like behavior.
- Gut Barrier and Stress: In a chronic stress experiment, scientists found that a protein called Reelin helped repair stress-induced damage in the gut lining, suggesting that gut health might influence mood regulation through immune signaling.
- Microbiome and Social Fear: Adolescents with social anxiety disorder carry specific gut bacteria that, when transferred to rats, trigger social fear and anxiety behaviors, highlighting the deep influence of gut microbes on emotional health.
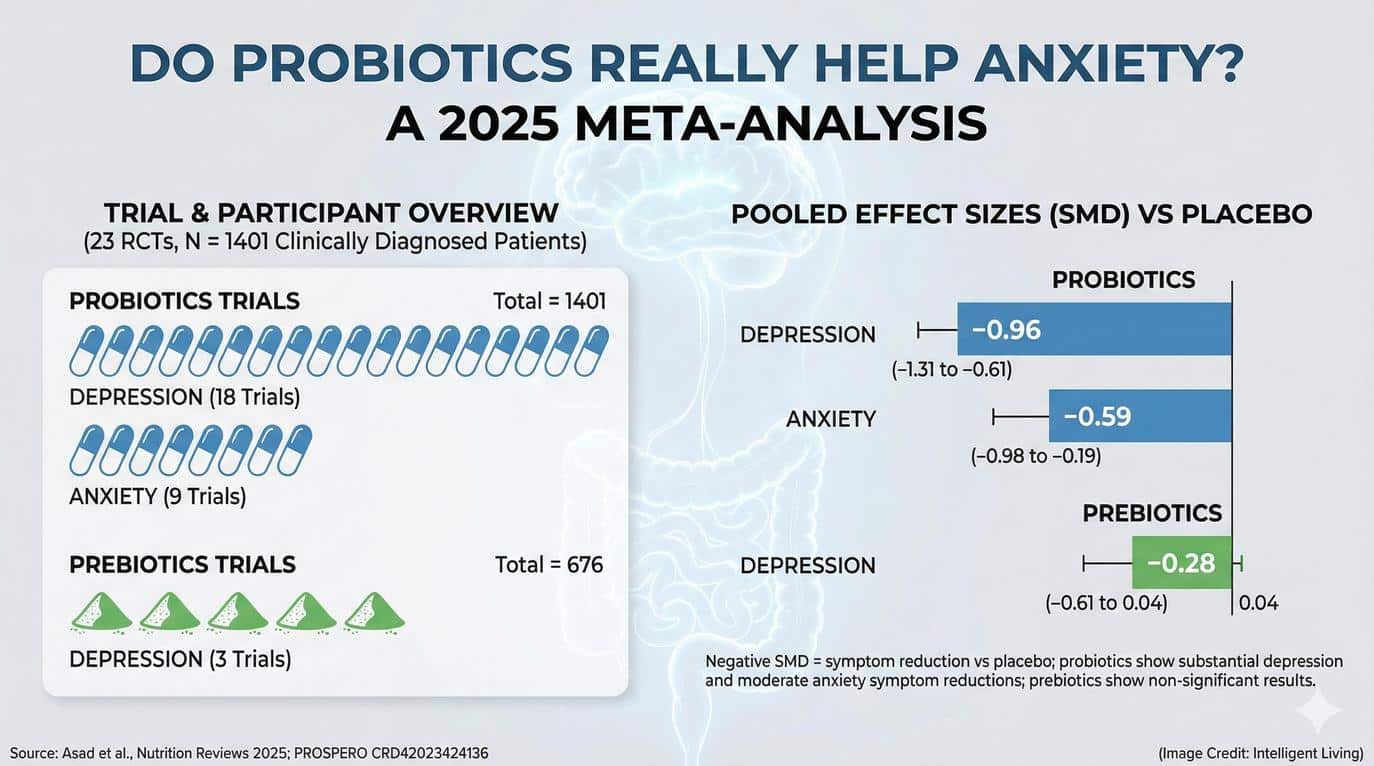
- Clinical Evidence Still Limited: Human trials involving probiotics show mixed results, with some studies reporting modest improvements in mood and anxiety symptoms but significant variation depending on strain and duration.
A Surprise Suspect in Anxiety: Immune Cells That Live in the Brain
Scientists traditionally viewed anxiety as a product of neurotransmitter imbalances and overactive neural circuits. Modern research has pivoted toward the brain’s immune system for deeper answers. Microglia represent a specialized class of immune cells comprising about ten percent of the brain’s total population. These cells are now showing a surprising amount of influence over emotional behavior. These cells constantly patrol neural tissue, clearing debris, regulating inflammation, and maintaining neural connections.
Recent evidence reveals that microglia directly shape anxiety-related responses by performing tasks far beyond simple cellular housekeeping. Evidence suggests that mood regulation depends partly on the interaction between brain immune cells and the neural pathways governing emotion and stress.
Systemic Inflammation and Reactive Microglia Signaling
Unlike neurons, microglia can shift rapidly between active and resting states, depending on cues from the body’s immune and hormonal systems. When inflammation rises, microglia tend to become more reactive, which can alter brain signaling in areas that govern fear and mood. Suspicions regarding the connection between inflammation and emotional processing have existed for years, yet these recent experiments provide the most direct evidence currently available.
The research also helps explain why conditions that trigger immune activation – such as infections, chronic stress, or autoimmune disorders – are often linked to increased anxiety and mood disturbances. Identifying microglia as central players in this process may eventually allow scientists to develop treatments that restore biological balance instead of merely suppressing symptoms.
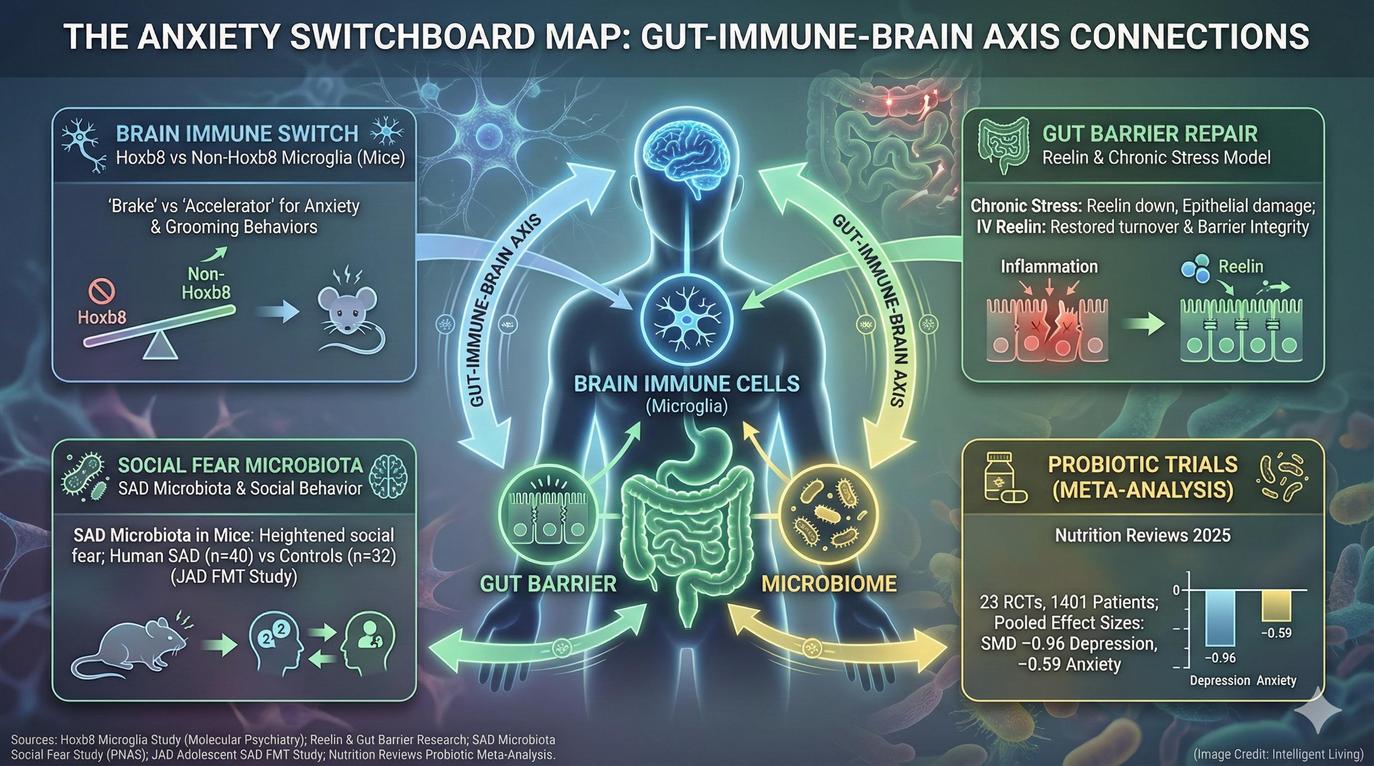
The “Accelerator and Brake” Model – What the Microglia Study Actually Showed
In their experiments, researchers examined two key subtypes of microglia distinguished by a genetic marker called Hoxb8. Roughly seventy-five percent of the microglia population in mice lack this gene, while the remaining twenty-five percent possess it. The non-Hoxb8 microglia acted as an accelerator, promoting anxiety-like grooming and defensive behaviors, whereas the Hoxb8-positive cells served as a brake, dampening those same tendencies.
To prove causation, the team performed cell transplantation experiments in mice that were bred to lack microglia. When they introduced Hoxb8 microglia into the animals’ brains, anxiety-related behaviors decreased. Conversely, adding non-Hoxb8 microglia heightened those behaviors. This dynamic functions as an internal balancing system—similar to a seesaw—where one group of microglia directly counteracts the effects of the other.
Anxiety may not be a simple circuit malfunction. Instead, it likely involves competing cellular signals that push and pull on neural activity. That insight also ties into emerging research showing that systemic inflammation and gut microbiome disturbances can indirectly influence microglia function through signaling molecules that cross the blood–brain barrier.
A diverse gut microbiome shields neurons from inflammatory stressors, reinforcing the biological connection between mental resilience and physical health. Together, these findings are shaping a more integrated understanding of mental health – one where the immune system acts not just as a defender but as a potential regulator of emotion.
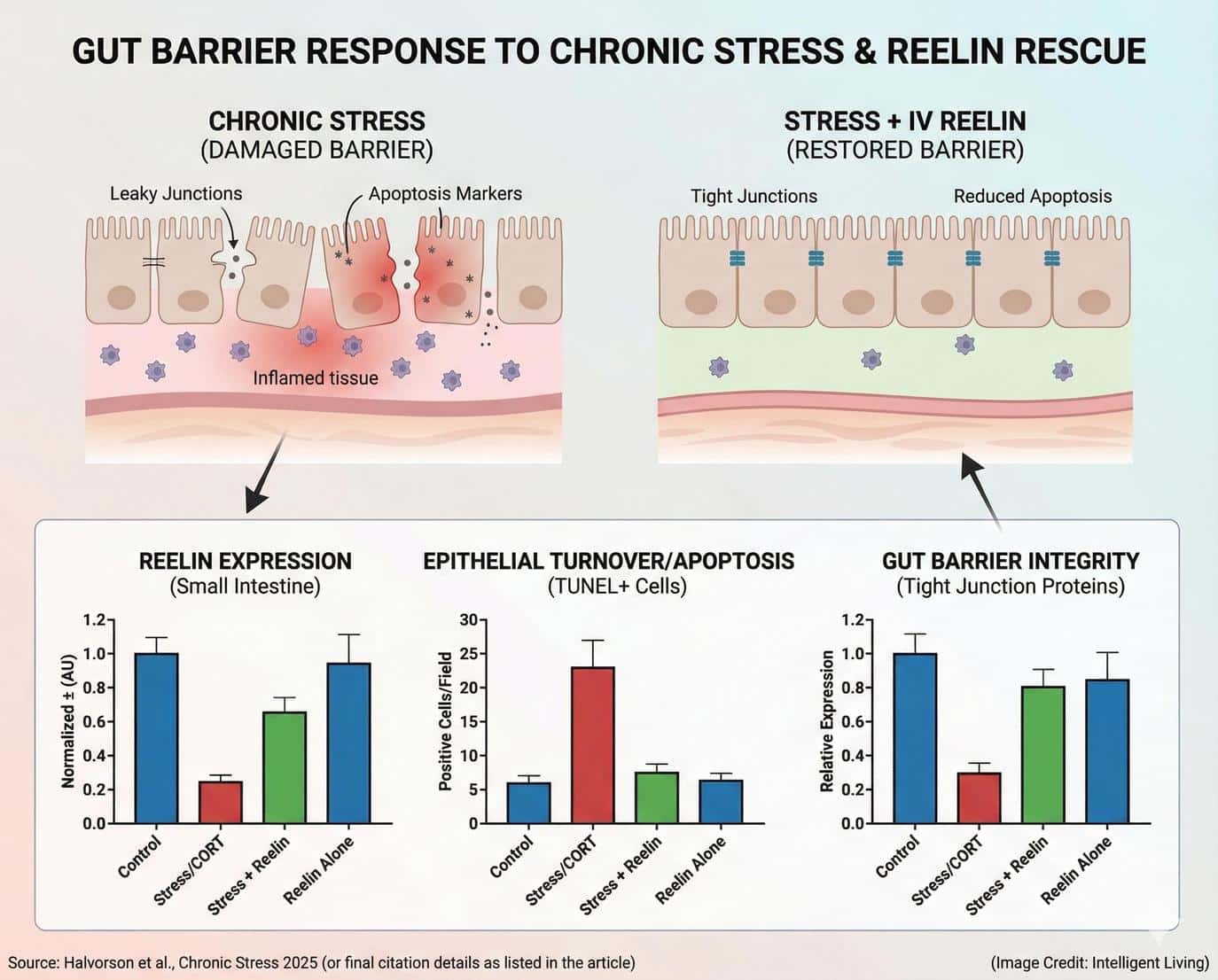
Depression Research is Also Circling the Gut – Reelin as a Barrier-Repair Clue
A second thread of research is revealing that the gut lining itself might be part of the body’s emotional circuitry. Scientists conducting preclinical trials observed how prolonged stress damages the intestinal barrier by significantly reducing essential protein levels. This process reduced levels of a protein called Reelin, which serves as a key factor in maintaining the health of epithelial cells forming the gut wall.
Following a single intravenous dose of recombinant Reelin, the animals’ gut tissue began a rapid recovery, showing normalized protein levels and markers of active cell renewal. Although these results were found in animals, they highlight a potential link between gut barrier health and mental resilience.
Chronic stress in humans is known to weaken digestive function, which can increase inflammation and alter the immune signals sent to the brain. Studies like this help explain how physical stress in the body may amplify emotional distress and why gut support might be crucial for overall well-being.
Reelin’s apparent ability to restore gut balance does not make it a treatment for depression or anxiety yet, but it provides an intriguing biological bridge. It suggests that interventions aimed at improving gut barrier function – through nutrition, stress management, or future therapeutics – might influence mood regulation indirectly. A stress-digestion loop illustrates how persistent worry disrupts motility and reshapes gut flora through a direct physiological link.

Social Anxiety May Leave a Footprint in the Gut Microbiome
Among adolescents diagnosed with social anxiety disorder, scientists have identified differences in the composition of their gut microbiota compared to their peers without anxiety. Researchers successfully transferred pooled gut bacteria from these adolescents into healthy rats to observe changes in emotional behavior. The animals began to exhibit anxiety-like behaviors and reduced interest in social interactions, suggesting that gut microbes might influence brain circuits linked to social fear.
Evidence reveals that social anxiety leaves a measurable biological footprint in the gut, where specific microbial patterns influence how individuals experience social stress. Research confirms that gut microbes from individuals with social anxiety increase social fear sensitivity without altering general anxiety or depressive behaviors. Researchers linked these physiological effects to alterations in immune function and oxytocin signaling. These two systems are already known to modulate social behavior and emotional bonding.
While scientists caution that this work is early stage, it opens a path toward precision-based microbiome therapies that focus on targeted bacterial strains rather than one-size-fits-all probiotic solutions. Specific microbiome patterns track with social and emotional traits across the human lifespan, suggesting a deep connection between microbes and wisdom.
Fiber-rich, minimally processed foods support a calmer nervous system by optimizing how gut bacteria influence anxiety.
Practical Strategies for Supporting Your Internal Switchboard
It is tempting to interpret these studies as a call to overhaul one’s diet or start taking probiotics, but the science is not yet settled. Human data remain limited, and most of the compelling results come from tightly controlled animal experiments. Still, the general message is clear: supporting gut and immune health supports brain health.
Clinical data suggests that consistent probiotic use modestly reduces stress and improves overall mood markers across various patient groups. However, outcomes vary widely, and not all products have been rigorously tested. Sugar-dense, ultra-processed dietary patterns often work against emotional stability, as specific foods trigger anxiety symptoms and heighten stress responses.
Evidence-Aligned Habits for Long-Term Nervous System Support
Eating a high-fiber diet, reducing chronic stress, and prioritizing restorative sleep promote a balanced microbiome and lower systemic inflammation. Focusing on these core habits promotes a balanced microbiome and significantly reduces systemic inflammation:
- High-fiber nutritional patterns to nourish beneficial gut microbes.
- Deliberate reduction of chronic stress through mindfulness or movement.
- Prioritization of restorative sleep to lower inflammatory markers.
- Consistent physical activity to strengthen the immune-gut connection.
Each of these lifestyle choices contributes to a more resilient nervous system. Managing stress, eating fiber, and prioritizing sleep represent evidence-aligned habits that support gut health while strengthening the nervous system. As researchers continue mapping these connections, the safest course is to focus on habits that strengthen overall resilience rather than chasing unverified supplements.

Science Behind the Holistic Mental Health Ecosystem
Scientific inquiry into the brain-immune-gut axis reveals that human emotions are not isolated within the skull. Instead, we are witnessing the birth of a new mental health map—one where the mind and body function as a single, continuous ecosystem. Brain immune cells, gut barrier integrity, and microbial diversity are all essential partners in emotional well-being.
Acknowledging this connection allows researchers to approach anxiety with more compassion and biological precision. The next wave of mental health innovation will likely focus on precision interventions that address these systems together rather than separately.
The most profound takeaway from this emerging research involves the power of biological balance. Supporting mental wellness now requires more than just managing thoughts; it necessitates a commitment to nourishing the physical systems that ground the nervous system. Options like fiber-rich nutrition, consistent stress management, or prioritizing sleep help stabilize the ‘Anxiety Switchboard.’
As scientists look toward the future, the realization that mental resilience is woven into human biology provides a hopeful path for those seeking a deeper sense of calm and clarity. Personalized functional medicine healthcare and studies on climate-related anxiety both underscore the intricate weaving of environment, biology, and emotion.
Essential Insights: Frequently Asked Questions on Anxiety and the Gut-Brain Connection
Are these scientific discoveries already being used in human medicine?
Evidence is compelling, but many of the recent breakthroughs involving microglia and gut barrier repair are currently based on animal models. Clinical trials in humans are ongoing to ensure these mechanisms translate safely and effectively into new therapies.
How does ‘leaky gut’ influence daily anxiety levels?
Weakened intestinal barriers can allow inflammatory markers to enter the bloodstream and eventually cross into the brain. Systemic inflammation can trigger the brain’s immune cells to become reactive, potentially amplifying feelings of worry and emotional tension.
Can specific probiotic strains target social anxiety?
Early research suggests that certain microbial patterns are linked to social fear, but there is no one-size-fits-all supplement yet. Scientists are working to identify precision-based bacterial strains that could specifically modulate the brain circuits governing social interaction.
Why are immune cells called microglia important for mood?
Microglia act as the brain’s internal regulators, clearing debris and managing inflammation. Recent studies show they can function like an ‘accelerator or brake’ for anxiety, directly influencing how we respond to stress.
What lifestyle changes best support the brain-immune switchboard?
Effective strategies include a high-fiber diet to support gut microbes, prioritizing restorative sleep to lower inflammation, and practicing stress-reduction techniques like mindfulness to keep the nervous system in balance.
